In the deepest parts of the ocean, below 13,100 feet (4,000 metres), the combination of high pressure and low temperature creates conditions that dissolve calcium carbonate, the material marine animals use to make their shells.
This zone is known as the carbonate compensation depth — and it is expanding.
This contrasts with the widely discussed ocean acidification of surface waters due to the ocean absorbing carbon dioxide from the burning of fossil fuels.
But the two are linked: because of rising concentrations of carbon dioxide in the ocean, its pH is decreasing (becoming more acidic), and the deep-sea area in which calcium carbonate dissolves is growing, from the seafloor up.
The transition zone within which calcium carbonate increasingly becomes chemically unstable and begins to dissolve is called the lysocline. Because the ocean seabed is relatively flat, even a rise of the lysocline by a few metres can rapidly lead to large under-saturated (acidic) areas.
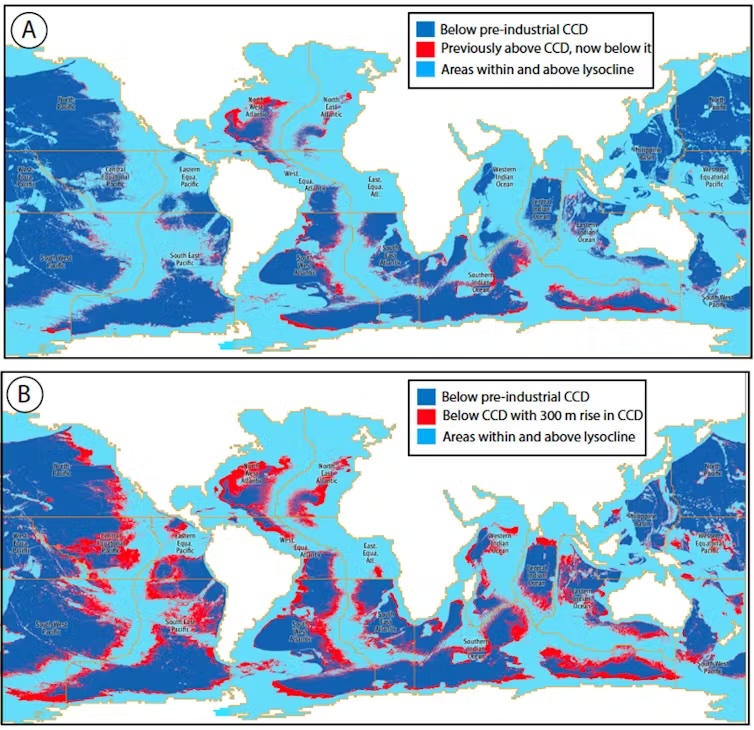
Our research showed this zone has already risen by nearly 100 metres since pre-industrial times and will likely rise further by several hundreds of metres this century.
Millions of square kilometres of ocean floor will potentially undergo a rapid transition whereby calcareous sediment will become chemically unstable and dissolve.
Expanding boundaries
The upper limit of the lysocline transition zone is known as the calcite saturation depth, above which seabed sediments are rich in calcium carbonate and ocean water is supersaturated with it. The calcite compensation depth is its lower limit, below which seabed sediments contain little or no carbonate minerals.
RELATED: Alaska’s rivers are turning bright orange and as acidic as vinegar as toxic metal escapes from melting permafrost
The area below the calcite compensation depth varies greatly between different sectors of the oceans. It already occupies about 41% of the global ocean. Since the industrial revolution, this zone has risen for all parts of the ocean, varying from almost no rise in the western Indian Ocean to more than 980 feet (300 m) in the northwest Atlantic.
If the calcite compensation depth rises by a further 980 feet, the area of seafloor below it will increase by 10% to occupy 51% of the global ocean.
Distinct habitats
For the first time, a recent study showed the calcite compensation depth is a biological boundary with distinct habitats above and below it. In the northeast Pacific, the most abundant seabed organisms above the calcite compensation depth are soft corals, brittle stars, mussels, sea snails, chitons and bryozoans, all of which have calcified shells or skeletons.
However, below the calcite compensation depth, sea anemones, sea cucumbers and octopus are more abundant. This under-saturated (more acidic) habitat already limits life in 54.4 million square miles (141 million square kilometres) of the ocean and could expand by another 13.5 million square miles (35 million sq/km) if the calcite compensation depth were to rise by 980 feet.
In addition to the expansion of the calcite compensation depth, parts of the ocean in low latitudes are losing species because the water is getting too warm and oxygen levels are declining, both also due to climate change.
Thus, the most liveable habitat space for marine species is shrinking from the bottom (rising calcite compensation depth) and the top (warming).
Island nations most affected
The exclusive economic zones of some countries will be more affected than others. Generally, oceanic and island nations lose more, while countries with large continental shelves lose proportionately less.
Bermuda’s EEZ is predicted to be the most affected by a 980-feet rise of the calcite compensation depth above the present level, with 68% of that country’s seabed becoming submerged below the lysocline. In contrast, only 6% of the US EEZ and 0.39% of the Russian EEZ are predicted to be impacted.
From a global perspective, it is remarkable that already 41% of the deep sea is effectively acidic, that half may be by the end of the century, and that the first study showing its effects of marine life was only published in the past year.
This edited article is republished from The Conversation under a Creative Commons license. Read the original article.

