New Delhi: The US Food and Drug Administration (USFDA) last week approved two breakthrough gene therapies — Casgevy by Vertex Pharmaceuticals and CRISPR Therapeutics, and Lyfgenia by Bluebird Bio — for sickle cell disease (SCD) in patients 12 years and older.
The development marks a milestone medical advancement in treating a debilitating disease that primarily affects red blood cells’ capacity to carry adequate oxygen across the body, with the use of innovative cell-based gene therapies.
Both approved products are made from patients’ own blood stem cells, which are modified, and are given back as a one-time, single-dose infusion as part of a hematopoietic (blood) stem cell transplant.
Casgevy utilises CRISPR/Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats-CRISPR associated) technology, a type of genome editing system.
Emmanuelle Charpentier and Jennifer Doudna were awarded the Nobel Prize in Chemistry in 2020 for discovering CRISPR/Cas9 genetic scissors, called one of the gene technology’s sharpest tools.
In India, which has the highest number of SCD carriers in the world, scientists associated with the Council for Scientific and Industrial Research-Institute of Genomics and Integrative Biology (CSIR-IGIB) have been working since 2018 to develop a gene therapy for SCD using the same technology.
“After showing proof of the therapy developed in human-induced pluripotent stem cells (a particular potent type of stem cell that normally only exists during early embryonic development), we are now in preclinical stage of the therapy’s trial,” Debojyoti Chakraborty, lead scientist of the project at CSIR-IGIB, told ThePrint.
The next step after the animal study, he said, is to start a phase-1 clinical trial for SCD patients in India, in partnership with the All India Institute of Medical Sciences (AIIMS) in Delhi and the department of science and technology after regulatory approvals for the therapy are in place.
Once available in the country, the therapy can be a boon for millions of SCD patients in India which this year saw the launch of the National Sickle Cell Anaemia Elimination Mission — targeting to eliminate the disease by 2047.
Also Read: New hope for HIV cure: Dual gene editing ‘eliminates’ infection, targets protein that helps virus enter body
How do the new therapies work?
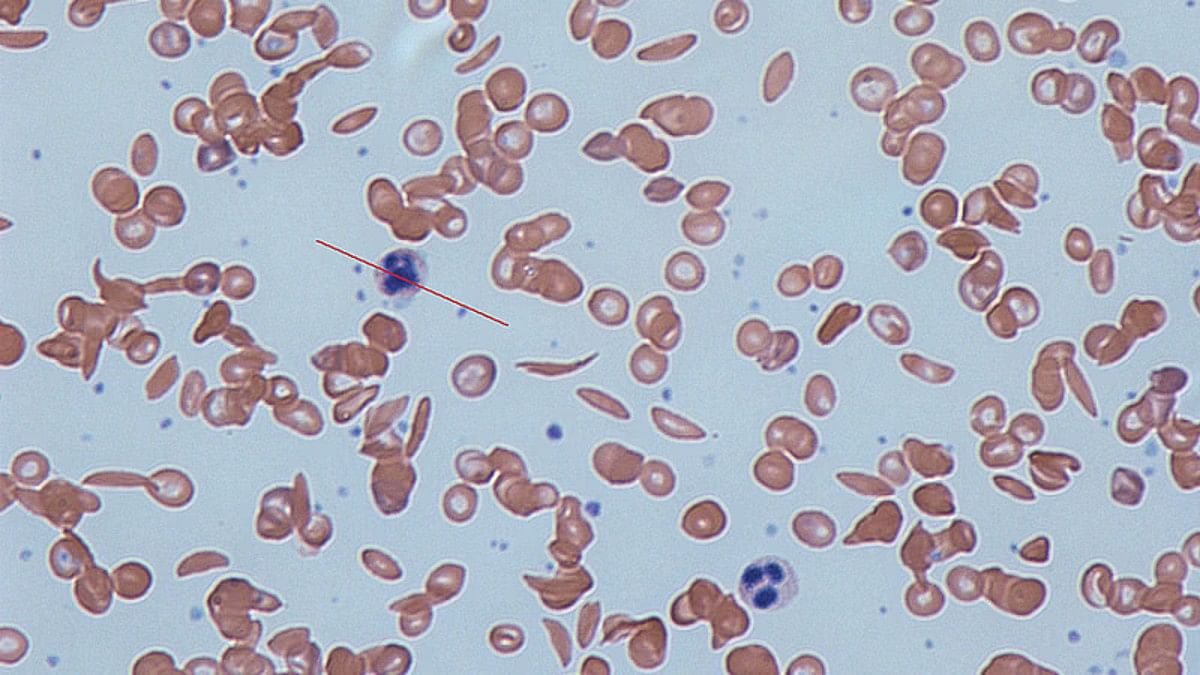
Sickle cell disease is a group of inherited blood disorders mainly caused by a mutation in haemoglobin, a protein found in red blood cells that delivers oxygen to the body’s tissues. This mutation causes red blood cells to develop a crescent or “sickle” shape.
These sickled red blood cells restrict the flow in blood vessels and limit oxygen delivery to the body’s tissues, leading to severe pain and organ damage called vaso-occlusive events (VOEs) or vaso-occlusive crises (VOCs).
The recurrence of these events or crises can lead to life-threatening disabilities and early death. The USFDA has approved both new treatments for use in SCD patients who are 12 years and older and have a history of VOCs.
In case of treatment with Casgevy, patients’ hematopoietic (blood) stem cells are modified by genome editing using CRISPR/Cas9 technology. This tool can be directed to cut DNA in targeted areas, enabling the ability to accurately edit (remove, add, or replace) DNA where it was cut.
The modified blood stem cells are then transplanted back into the patient where they engraft (attach and multiply) within the bone marrow and increase the production of foetal haemoglobin (HbF), a type of haemoglobin that facilitates oxygen delivery.
In patients with SCD, increased levels of HbF prevent the sickling of red blood cells.
Lyfgenia is also a cell-based gene therapy but uses a lentiviral vector (gene delivery vehicle) for genetic modification.
With this therapy, the patient’s blood stem cells are genetically modified to produce HbAT87Q, a gene-therapy derived haemoglobin that functions similarly to haemoglobin A, which is the normal adult haemoglobin produced in persons not affected by sickle cell disease.
Red blood cells containing HbAT87Q have a lower risk of sickling and occluding blood flow. These modified stem cells are then delivered to the patient.
Both products are made from the patients’ own blood stem cells, which are modified, and are given back as a one-time, single-dose infusion as part of a hematopoietic (blood) stem cell transplant.
Prior to treatment, a patients’ own stem cells are collected, and then the patient must undergo myeloablative conditioning (high-dose chemotherapy), a process that removes cells from the bone marrow so they can be replaced with the modified cells in Casgevy and Lyfgenia.
But these therapies — though highly effective as proven in clinical trials — are not without side-effects.
In case of Casgevy, the most common side effects were low levels of platelets and white blood cells, mouth sores, nausea, musculoskeletal pain, abdominal pain, vomiting, febrile neutropenia (fever and low white blood cell count), headache and itching.
The most common side effects attached with Lyfgenia included stomatitis (sores on the lips, mouth and throat), low levels of platelets, white blood cells and red blood cells, and febrile neutropenia. Hematologic malignancy (blood cancer) also occurred in patients treated with Lyfgenia.
Patients who receive Casgevy or Lyfgenia will be followed in a long-term study to evaluate each product’s safety and effectiveness, according to the USFDA.
How can India gain from novel therapies?
India is among the countries with the highest burden of the disease globally, second only to Nigeria, and mostly tribal people are afflicted with it, with some communities reporting as high as 40 per cent of the population as affected.
The states reporting the highest number of patients include Madhya Pradesh, Gujarat, Maharashtra, Chhattisgarh and Tamil Nadu, among others.
The disease is marked in patients with symptoms such as acute episodes of pain, acute chest syndrome including chest pain, high temperature (fever), shortness of breath and infections. Many patients of the condition do not survive beyond five years and the average lifespan of these patients is about 40-42 years.
Currently, only symptomatic treatment is carried out for most patients with SCD to manage their symptoms. The only curative treatment, bone marrow transplant, is complicated, and can be offered mostly to paediatric patients, provided they have donors with matching biological specifications.
Some experts pointed out that best results from bone marrow transplants are only from matched siblings of age younger than 13 years, and that too has its own set of challenges.
“Transplant comes with its subset of complications and 10 per cent mortality and morbidity,” said Dr Rahul Bhargava, principal director of haematology and bone marrow transplant at Fortis Memorial Research Institute in Gurugram.
Therefore, he added, gene therapy, if affordable, can serve a large number of people irrespective of age and its complications. “Bone marrow transplant is more labour-intensive while gene therapy can easily be scaled up to serve a large number of patients,” he asserted.
(Edited by Nida Fatima Siddiqui)
Also Read: All about India’s 1st ‘living drug’ that uses patient’s own genetically engineered cells to fight cancer