New Delhi: Wearable devices such as smartwatches and smart rings that claim to measure blood glucose levels in real-time without piercing the skin may seem convenient for people with diabetes. However, the US Food and Drug Administration (FDA) has warned that these devices may show inaccurate and misleading results.
The FDA issued a safety communication last week to alert consumers, patients, caregivers, and healthcare providers of risks associated with using smartwatches or smart rings that claim to measure blood glucose levels non-invasively.
Blood sugar management is considered the cornerstone of managing both type-1 and type-2 diabetes.
In India, where an estimated 101 million people have diabetes, many smartwatches, mostly made by smaller technology players, claim to record and monitor blood glucose levels in real time and several people use them, according to medical experts.
However, these devices are not approved by the Central Drugs Standard Control Organisation — the country’s drug and medical device regulatory authority.
“For people with diabetes, inaccurate blood glucose measurements can lead to errors in diabetes management, including taking the wrong dose of insulin, sulfonylureas, or other medications that can rapidly lower blood glucose,” the FDA said in its safety communication issued on 21 February.
The FDA also clarified that these smartwatches are different from smartwatch applications that display data from FDA-authorised blood glucose measuring devices that pierce the skin, such as continuous glucose monitoring devices (CGMs). CGMs include a sensor that is inserted under the skin of a person with diabetes and records glucose levels and sends data to a connected software.
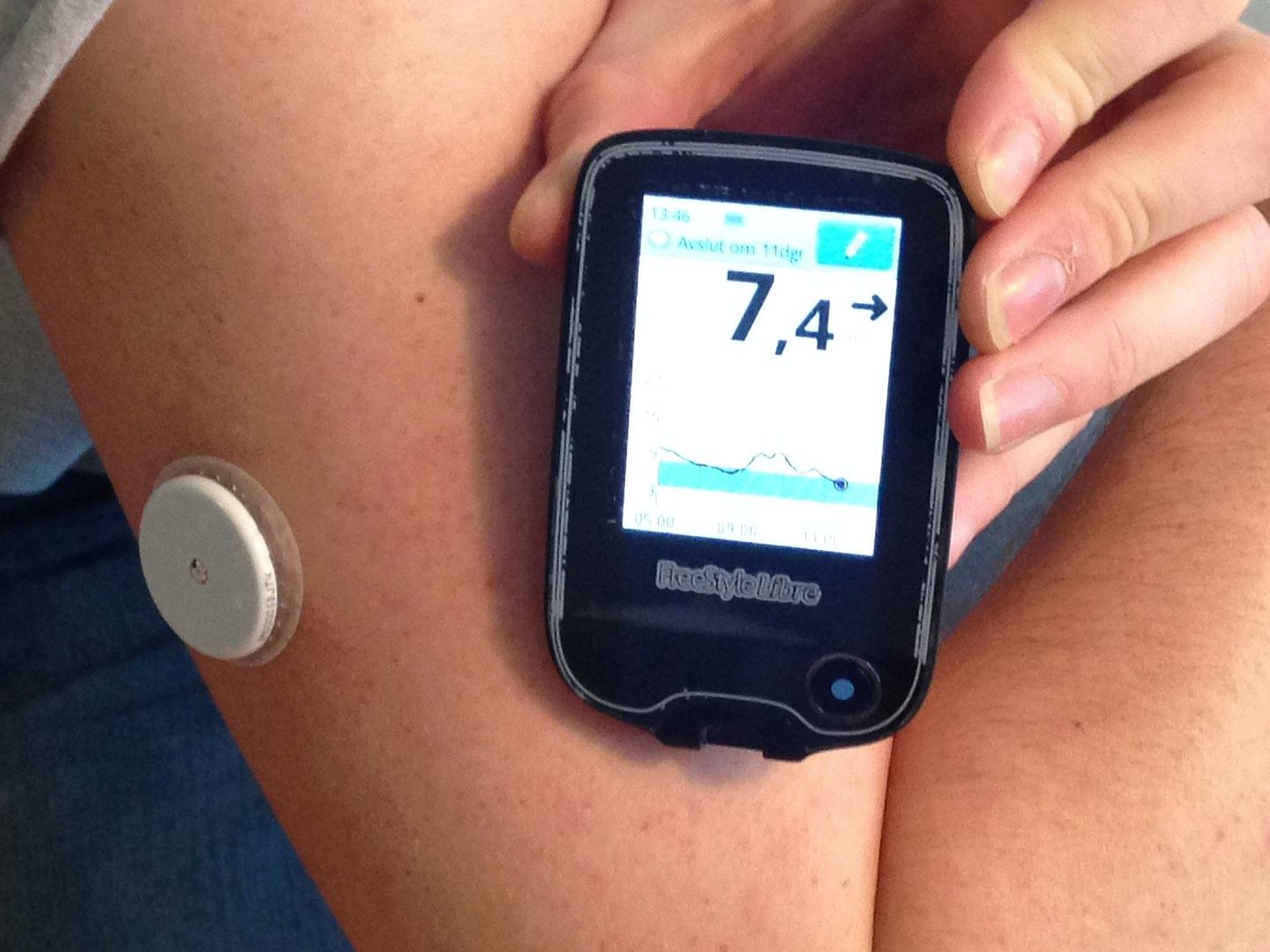
The FDA has not authorised, cleared, or approved any smartwatch or smart ring that is intended to measure or estimate blood glucose values on its own, the agency has also said.
According to media reports, technology giants like Apple and Samsung have also been planning to launch smartwatches soon that can track blood glucose levels just like they measure blood pressure, blood oxygen and heart rate levels using skin sensing technology.
Currently, the smartwatches made by Apple, Samsung, and Google-owned FitBit support integrations with FDA-authorised CGMs, which are also wearable devices that use needles to read blood sugar levels. These integrations allow users to view data from connected CGMs that have companion smartphone apps.
Dr Rajeev Gupta, director, internal medicine at Delhi’s C.K. Birla Hospital, said that the development of non-invasive glucose analysis using sensor technology has been vigorously pursued by various companies.
“While successful implementation holds the potential to significantly improve diabetic control and prevent long-term complications, current optical and transdermal approaches face significant limitations,” he said.
Gupta stressed that these methods often suffer from inaccurate and unstable readings, potentially leading to unintended hypoglycemia — when erroneous results prompt patients to consume glucose-lowering medications — and other complications.
“The FDA has recommended against using commercially available glucose watches and rings due to concerns regarding the accuracy, long-term safety, and stability,” Gupta told ThePrint.
He underlined that further research and development are necessary before these technologies can be considered reliable and safe for clinical use.
Also Read: ICMR calls for development of local drugs for rare diseases, offers assistance in clinical research
‘Dangerous consequences’
Sellers of smartwatches and smart rings claim that their devices measure blood glucose levels using non-invasive techniques without requiring people to prick their fingers or pierce the skin, the FDA said.
“These smartwatches and smart rings are manufactured by dozens of companies and sold under multiple brand names,” it said, adding that the warning applies to any smartwatch or smart ring that claims to measure blood glucose without piercing the skin — regardless of manufacturer or brand.
According to top diabetologist Dr V. Mohan, chairman of Dr Mohan’s Diabetes Specialities Centre in Chennai, he had been associated with the field trials to assess the accuracy of a glucose watch by biotechnology company Cygnus in the 2000s. The company was later dissolved.
“The device was not accurate. We did some trials in India and found huge errors in the measurement of glucose levels at that time. It was attributed to the hot, humid climate in India with excessive sweating, which made these glucose watches inaccurate and unreliable,” he recalled.
However, soon, even abroad, these devices were withdrawn for some time, and for many years thereafter, there was a lull in the production of these smartwatches and rings for estimating glucose levels, Mohan told ThePrint.
Although there has been some talk of major players, such as Apple, getting into the business, the FDA warning should work like a clear red flag for diabetics, said the doctor.
Pointing out the consequences of the devices showing inaccurate results, he said, “A person with diabetes driving a vehicle may have dangerously low glucose levels, but the device may show a normal reading and this could lead to catastrophic consequences.”
Mohan highlighted that even with the current CGMs approved for clinical use and used widely, there is a lag time between blood glucose estimation and the interstitial glucose readings, as these devices measure sugar levels in interstitial fluid or fluid around the cells, rather than in blood.
This lag could be around 20-30 minutes, which means what the CGM shows is the blood glucose that was present 20-30 minutes earlier, and although this gap is being narrowed, there is still a gap between blood glucose and interstitial glucose measurements, he explained.
While in practice getting a CGM still gives valuable information, such as time and range, wearing it as a watch or a ring, unless it is very accurate, can lead to dangerous situations, Mohan said.
“One does wish that ultimately the technology improves to a point that the wearable devices can measure blood sugar level precisely, just as fitness devices measure blood pressure, heart rate and step count, among others,” he said.
Until that time, he added, these instruments were to be considered as under development and not yet ready for clinical use by millions of people all over the world.
Even if such devices are approved elsewhere, Mohan warned, there have to be separate field trials for warm, tropical countries like India, where skin colour and sweating may affect their performance, and the devices need to prove their effectiveness under the local conditions.
(Edited by Richa Mishra)
Also Read: US FDA approves ‘Xolair’ injection — all about the drug that can help treat severe food allergies

